Patient Safety and Laboratory Efficiency

Lizette Caballero – University of California, San Francisco (UCSF), Senior Supervisor of the Blood and Marrow Transplant Laboratory

Always focused on quality and innovation, Lizette Caballero, who served as Senior Supervisor at UCSF’s Blood and Marrow Transplant Laboratory for nearly 10 years and is currently a CAR-T Cell Therapy Specialist at Janssen Pharmaceutical Companies of Johnson & Johnson, sought a safer, more hygienic, and efficient method for thawing cryopreserved hematopoietic cells during her time at UCSF.
Challenges with Water Baths
During the processing of blood components, mechanical forces can cause small cracks (especially concerning in frozen bags), allowing pathogens to enter. This risk of contamination from microorganisms in water is a recurring concern, so significant that nearly all of Germany has eliminated the use of water baths. Furthermore, water baths require constant maintenance, including water changes after each use, which consumes the time of already overburdened laboratory teams.
In the United States, water baths set at 37°C are widely used to thaw cellular therapy products. This method involves manually immersing the product in temperature-controlled water, handling bags or vials to ensure even thawing. However, there is widespread concern about microbial contamination, which can put vulnerable patients at higher risk.

Innovative Solution: Barkey Plasmatherm
Lizette Caballero discovered the Barkey Plasmatherm, a dry heating device that thaws products between two water-filled pads, with the water replaced only once a year. This device eliminates the challenges associated with water baths, providing a safer and more efficient thawing method.
Key Advantages of the Barkey Plasmatherm:
- Enhanced patient safety by virtually eliminating the risk of pathogen contamination from water.
- Process standardization, removing variations associated with manual handling.
- Time savings for the laboratory, reducing maintenance by approximately 45 minutes per day—equivalent to 196 hours or roughly €10,000 per year.

Comparative and Validation Studies
Always data-driven, Caballero conducted comparative studies between the Plasmatherm and the 37°C water bath. On June 1, 2020, she presented the study titled “Thawing Cryopreserved Apheresis Products: Comparison of Barkey Plasmatherm Dry Heating Device and 37°C Water Bath” at the International Society for Cell and Gene Therapy, held virtually in Paris.
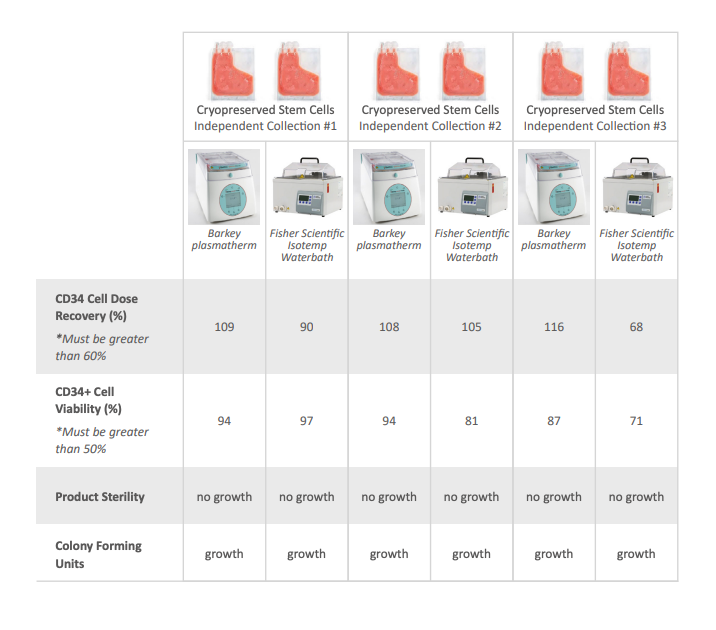
The results showed that both methods achieved similar quality criteria in terms of CD34+ cell dose recovery, viability, and sterility. However, the Plasmatherm eliminated microbial contamination risks and reduced maintenance time.
Patient Impact
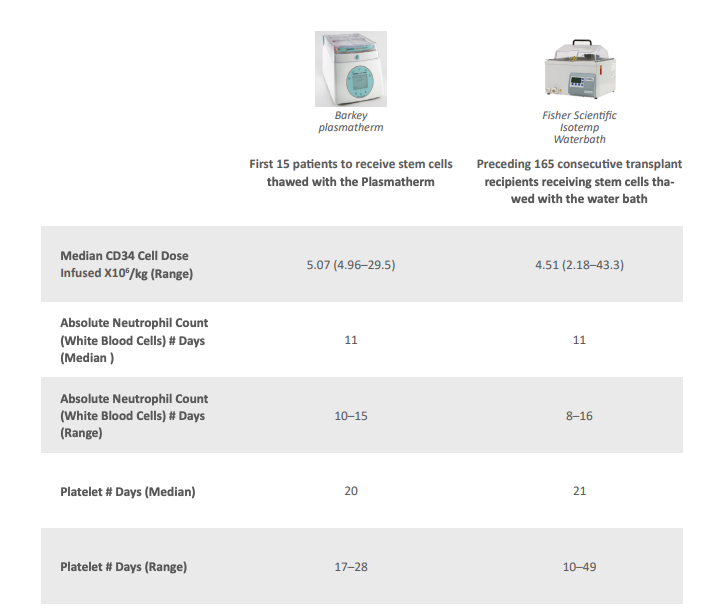
Caballero also evaluated clinical outcomes in 15 patients who received cells thawed using the Plasmatherm, compared to 165 previous patients who received cells thawed with a water bath. The results demonstrated equivalence in neutrophil and platelet recovery, confirming the efficacy of the new method.
Key Measurements:
- Recovery and viability of CD34+ cells: Both methods met release criteria.
- Neutrophil and platelet recovery time: No significant differences between methods.
Conclusion
Caballero emphasized that patient safety must always be a priority in healthcare. The Barkey Plasmatherm not only enhances safety in handling stem cells but also improves standardization and efficiency in the laboratory setting. This innovation translates into better care delivery and more satisfied laboratory teams due to reduced repetitive tasks.
As Caballero states:
“I am excited to leverage innovation to advance the field of stem cell transfusion. I will continue improving healthcare and sharing my expertise.”
